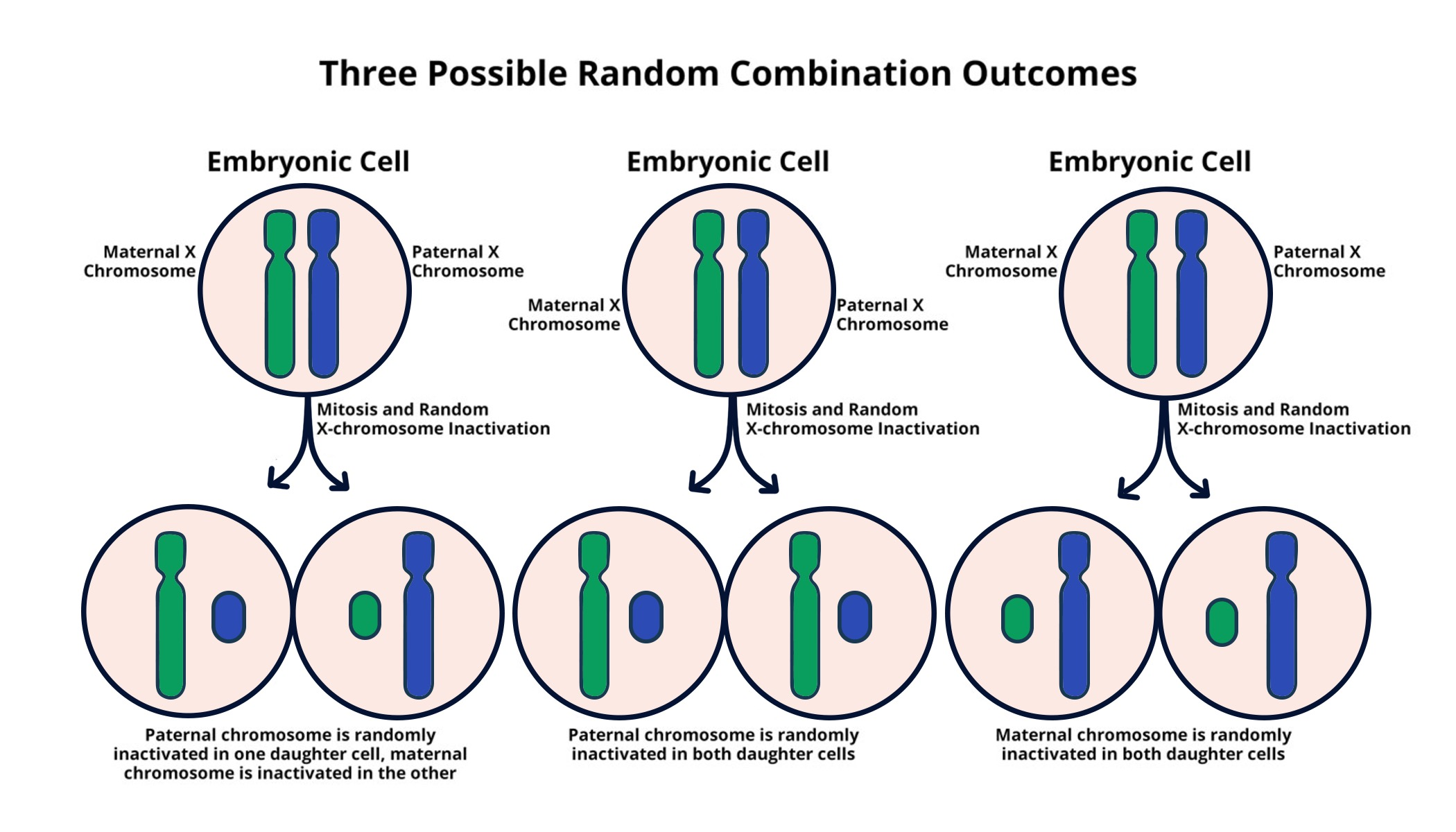
X chromosome inactivation is a fascinating biological process essential for balancing gene expression between male and female cells. In females, the presence of two X chromosomes means that one must be turned off to prevent an overload of gene products, a phenomenon known as chromosomal silencing. This intricate mechanism plays a vital role in the development of various genetic disorders, including Fragile X Syndrome and Rett Syndrome, both linked to mutations on the X chromosome. Ongoing research, particularly from Jeannie T. Lee’s lab at Harvard Medical School, suggests that understanding and potentially reversing X chromosome inactivation could pave the way for innovative gene therapy treatments. These breakthroughs not only highlight the significance of X chromosome biology but also open new avenues for providing relief to individuals affected by X-linked genetic conditions.
The process known as X chromosome silencing is crucial for maintaining genetic balance in humans, particularly in females who have dual X chromosomes. To ensure that cells operate efficiently, one of these X chromosomes is inactivated, a strategy that helms genetic stability. This mechanism is closely associated with disorders like Fragile X and Rett syndromes, arising from mutations within the X chromosome’s genes. The groundbreaking research from Vaughan Lee and her team at Harvard Medical School reveals promising potential for gene therapy aimed at unsilencing inactivated genes, which could lead to novel treatments. Examining the biological nuances of how the X chromosome operates provides critical insights into the management of various genetic conditions.
Understanding X Chromosome Inactivation Mechanisms
X chromosome inactivation (XCI) is a fascinating process that demonstrates how biology adapts to challenges presented by genetic variation between sexes. In females, having two X chromosomes leads to the necessity of silencing one copy to maintain gene dosage balance with males, who possess only one X chromosome. This intricate biological mechanism involves a sophisticated interplay of genes and RNA molecules, notably the Xist gene, which initiates the inactivation process through chromosomal silencing. By understanding the underlying mechanics of XCI, researchers can delve deeper into potential therapies for X-linked genetic disorders.
The Lee lab’s pioneering research provides crucial insights into the biochemical changes occurring during XCI. This process is analogous to a jelly-like substance surrounding chromosomes that helps maintain their structural integrity while allowing for selective silencing of one X chromosome. The study reveals that mutations leading to conditions such as Fragile X Syndrome can potentially be countered by manipulating this chromosomal silencing, offering hope for innovative gene therapy techniques. The ongoing exploration into the factors influencing XCI could propel forward our ability to treat various genetic disorders, making it a key area of focus for future research.
The Impact of Chromosomal Silencing on Fragile X Syndrome
Fragile X Syndrome (FXS) is one of the most common inherited causes of intellectual disability, closely tied to mutations on the X chromosome. The research by Jeannie Lee and her colleagues sheds light on how the mechanisms of X chromosome inactivation can impact the expression of genes associated with FXS. By elucidating how inactivated X chromosomes can harbor healthy copies of genes that are otherwise rendered ineffective due to mutations, Lee’s work highlights the potential for therapeutic interventions that could reactivate these silenced genes.
In practical terms, the possibility of ‘unsilencing’ the inherited gene responsible for FXS opens a door to novel treatments and gene therapies that could radically alter the lives of those affected by this condition. As researchers like Lee look to refine their approaches, the utilization of gene therapy could lead to groundbreaking strategies that not only restore function to inactivated X-linked genes but also minimize adverse effects on healthy genes. This approach could provide a new lease on life for countless individuals with Fragile X Syndrome.
Advancements in Gene Therapy for Rett Syndrome
Rett Syndrome, a neurodevelopmental disorder also linked to mutations on the X chromosome, has become another focus of interest in the context of chromosomal silencing research. With insights gained from studying X chromosome inactivation, scientists are exploring the potential for gene therapy to correct or compensate for the defective genes causing Rett Syndrome. By leveraging the mechanisms underlying XCI, researchers aim to develop strategies that target the silenced gene and reactivate its expression, providing much-needed relief for patients.
The work conducted at Harvard Medical School emphasizes not only the importance of fundamental research but also its therapeutic implications. Gene therapy targeting Rett Syndrome could change the landscape of treatment options available. Lee’s findings suggest that bringing mutated genes into active status may help restore neural function in patients, showcasing a promising avenue to address the challenges posed by rare genetic disorders. This innovative perspective highlights the urgent need for clinical trials and further investigation into the safety and efficacy of such interventions.
Role of Harvard Medical School in Genetic Research
Harvard Medical School has established itself as a leader in genetic research, particularly in understanding complex mechanisms like X chromosome inactivation. With researchers like Jeannie Lee pushing the boundaries of knowledge in this crucial field, the institution plays a fundamental role in uncovering the biological secrets that underlie genetic diseases. By fostering an environment that encourages the convergence of basic science and translational research, Harvard is setting the stage for breakthroughs that could ultimately lead to practical treatments for patients suffering from X-linked disorders.
Collaborations across disciplines at Harvard Medical School further enhance the potential for innovative discoveries in genetics. By integrating insights from molecular biology, clinical research, and therapeutic development, the school’s initiatives aim to bridge the gap between laboratory research and real-world impacts. Such interdisciplinary efforts are crucial for developing comprehensive strategies for diseases like Fragile X Syndrome and Rett Syndrome, transforming how we understand and treat genetic conditions moving forward.
Implications of Chromosomal Silencing in Genetic Disorders
The implications of chromosomal silencing through mechanisms such as X chromosome inactivation extend far beyond basic biology; they offer critical insights into genetic disorders. Understanding how certain genes are silenced allows scientists to formulate targeted treatments that could alleviate the symptoms associated with X-linked diseases. Conditions like Fragile X Syndrome highlight the complexity of gene expression and the need for interventions that can selectively reactivate silenced genes while preserving the integrity of healthy gene function.
Moreover, emerging research suggests that advancements in gene therapy could leverage chromosomal silencing to improve outcomes for patients with a variety of genetic disorders. The knowledge gained from XCI could empower researchers to develop novel therapeutic approaches that specifically address the root causes of these conditions. As science continues to unravel the intricacies of genetic regulation, there exists a renewed hope for treating diseases once thought untreatable.
The Future of Research in Gene Therapy
The future of research in gene therapy is poised for unprecedented developments, particularly in the realm of X-linked genetic disorders. As studies like those conducted in Jeannie Lee’s lab gain momentum, the trajectory of gene therapy may shift toward radical new paradigms for treating conditions like Fragile X Syndrome and Rett Syndrome. With ongoing research focused on mechanisms of chromosomal silencing, the potential for safe and effective therapies grows more tangible, promising to redefine the lives of individuals afflicted by these genetic diseases.
Innovative approaches that disrupt the status quo of gene expression hold the key to unlocking therapeutic solutions. By harnessing the power of biologically-based mechanisms like X chromosome inactivation, future researchers can develop precise drug formulations aimed at gene reactivation. This avant-garde trajectory ensures that patients may soon investigate viable options to counter genetic disorders, bringing scientists closer to fulfilling their ultimate mission of enhancing human health through advanced genetic therapies.
Advancements in Understanding Chromosomal Architecture
Recent advancements in understanding chromosomal architecture have shed light on the complex interactions that govern gene expression, including X chromosome inactivation. Studies have shown that the physical structure of chromosomes plays a pivotal role in mediating gene activity and silencing. Scientists now know that the spatial organization of chromosomes within the nucleus can impact how genes are expressed, particularly in females with two X chromosomes, revealing the elegant mechanisms that cells use to maintain balance between gene dosage and functionality.
This exploration of chromosomal architecture encourages researchers to look beyond traditional gene therapy approaches. By integrating knowledge of structural biology with genetic engineering, future therapeutic strategies may involve not only direct gene manipulation but also modulation of the spatial and physical context of chromosomal regions. Such a holistic view could transform the way we approach the treatment of X-linked disorders, providing a framework for innovative interventions that address the underlying complexities of genetic expression.
The Interplay of Genetics and Environmental Factors
An emerging area of research is the interplay between genetics and environmental factors in the expression of diseases linked to the X chromosome. It is becoming increasingly clear that while genetic mutations set the stage for conditions like Fragile X Syndrome and Rett Syndrome, environmental influences may modulate how these genetic factors express themselves in individuals. Thus, understanding the environmental aspects can provide critical context for developing effective therapeutic approaches.
Future studies that delve into how environmental variables interact with genetic predispositions could yield new insights into treating genetic disorders. For example, combining gene therapy with lifestyle interventions or environmental modifications may enhance treatment effectiveness. As researchers continue to unravel these complexities, the potential for more personalized treatment approaches becomes evident, ultimately leading to better outcomes for individuals affected by X-linked genetic conditions.
The Role of Funding in Advancing Genetic Research
Funding plays a significant role in advancing genetic research, particularly in specialized areas such as chromosomal inactivation and gene therapy. Generous support from institutions like the National Institutes of Health has allowed labs like Jeannie Lee’s to pursue groundbreaking studies over the years. This financial backing is crucial for facilitating the experimental work necessary to understand complex biological questions and translate them into actionable therapies.
As the field of genetic research evolves, the need for sustained investment becomes ever more crucial. Continued funding enables researchers to explore innovative approaches and refine their methodologies, ultimately leading to new treatments for diseases like Fragile X Syndrome and Rett Syndrome. Advocating for increased funding in genetic research will be vital to ensure that invaluable discoveries continue to thrive, paving the way for the next generation of medical breakthroughs.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in understanding conditions like Fragile X Syndrome?
X chromosome inactivation is a biological process that occurs in females where one of the two X chromosomes is silenced to ensure equal gene dosage between sexes. This is crucial in understanding conditions such as Fragile X Syndrome, where mutations on the X chromosome lead to intellectual disability. Understanding the mechanics of this process can help develop gene therapy approaches to reactivate the healthy gene.
How does the process of X chromosome inactivation affect gene expression related to Rett Syndrome?
During X chromosome inactivation, the active X chromosome silences the other. In Rett Syndrome, an X-linked disorder, understanding X inactivation helps researchers investigate ways to unsilence healthy genes that may be both inactive and mutated. This could lead to new treatments that restore function without affecting other crucial genes.
Can gene therapy be used to target X chromosome inactivation in diseases like Fragile X Syndrome and Rett Syndrome?
Yes, gene therapy has the potential to target X chromosome inactivation. By developing techniques to free inactivated genes, researchers can possibly restore function to genes affected by mutations linked to disorders such as Fragile X Syndrome and Rett Syndrome, which could transform patient outcomes.
What role does chromosomal silencing play in diseases linked to mutations on the X chromosome?
Chromosomal silencing, specifically through X chromosome inactivation, plays a critical role in diseases linked to X chromosome mutations. In disorders like Fragile X Syndrome, mutations may exist on one X chromosome, and understanding how X inactivation occurs can provide insights into therapeutic strategies aiming to reactivate the healthy gene.
What breakthroughs in X chromosome inactivation research have been made at institutions like Harvard Medical School?
Research at Harvard Medical School, particularly in Jeannie T. Lee’s lab, has uncovered fundamental insights into X chromosome inactivation. The lab’s studies reveal how the Xist RNA molecule modifies the chromatin structure, enabling potential strategies for gene therapy aimed at conditions like Fragile X Syndrome and Rett Syndrome.
How can understanding X chromosome inactivation lead to potential therapeutic approaches for genetic disorders?
By comprehensively studying X chromosome inactivation, researchers are uncovering mechanisms that explain how mutations on one X chromosome can be rendered inactive. Innovations from these findings may lead to therapeutic strategies that restore the function of the healthy gene in genetic disorders like Fragile X Syndrome and Rett Syndrome.
What is the significance of Xist in the context of X chromosome inactivation?
Xist is a key RNA molecule in X chromosome inactivation. It plays a central role by coating the inactive X chromosome and altering its chromatin environment, which ultimately leads to the silencing of gene expression. Understanding Xist is critical for developing gene therapy approaches targeting genetic disorders like Fragile X Syndrome.
What are the challenges in unsilencing inactivated X chromosomes for therapeutic purposes?
Unsilencing inactivated X chromosomes presents challenges, primarily ensuring that the correct genes are reactivated without affecting healthy genes. Ongoing research is focusing on optimizing techniques to selectively restore function to mutated genes in disorders like Fragile X Syndrome and Rett Syndrome while minimizing side effects.
| Key Concepts | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, while males have one. Females must inactivate one X to balance gene expression. |
| Role of Xist RNA | A gene on the X chromosome produces Xist, which alters the properties of the surrounding chromosomal substance. |
| Research Significance | Findings from Jeannie T. Lee’s lab could help treat conditions like Fragile X and Rett syndrome by targeting inactivated genes. |
| Current Research Directions | Lee’s lab is developing methods to unsilence X-linked genes and plans to move toward clinical trials. |
Summary
X chromosome inactivation is a crucial biological process that enables female mammals to manage the unequal dosage of X-linked genes. Recent research led by Jeannie T. Lee highlights the mechanisms of this inactivation, focusing on the role of Xist RNA and a gelatinous substance surrounding chromosomes. Understanding these processes not only resolves longstanding questions in molecular biology but also paves the way for innovative therapies targeting genetic conditions caused by mutations on the X chromosome. With promising advancements potentially leading to treatments for Fragile X syndrome and Rett syndrome, the implications of this research are significant for genetics and medical science.